

Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site and are used with permission.
Gracilinanus agilis (Burmeister 1854) Image Gallery
TAX: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Didelphimorphia; Family Didelphidae; Subfamily Didelphinae, Tribe Monodelphini (Myers et al 2006, Gardner 2007). The genus Gracilinanus was defined by Gardner & Creighton 1989. There are six known species according to the latest revision (Gardner 2007) one of which is present in Paraguay. The generic name Gracilinanus is taken from Latin (gracilis) and Greek (nanos) meaning "slender dwarf", in reference to the slight build of this species. The species name agilis is Latin meaning "agile" referring to the nimble climbing technique of this species. The species is monotypic, but Gardner (2007) considers it to be composite and in need of revision. Furthermore its relationship to the Brazilian coastal Atlantic Forest species Gracilinanus microtarsus needs to be examined, with some authorities suggesting that the two may be at least in part conspecific. The description of the cryptic and hitherto unnoticed genus Cryptonanus by Voss, Lunde & Jansa (2005) confused the situation yet further. This species is widely sympatric with Cryptonanus chacoensis with which it has been consistently confused in the literature in the past. A deliberately cautious approach to the species description has been adopted here to avoid muddying the waters yet further, and every effort has been made to quote references that refer unequivocally to Gracilinanus agilis and not Cryptonanus. Synonyms adapted from Gardner (2007):
Didelphys [(Grymaeomys)] agilis Burmeister 1854:139. Type locality "Lagoa Santa", Minais Gerais, Brazil.
Marmosa beatrix O.Thomas 1910:502: Type locality "Ipu", Ceará, Brazil.
[Didelphys (Grymaeomys)] agilis Matschie 1916:270. Name combination.
[Didelphys (Grymaeomys)]beatrix Matschie 1916:270. Name combination.
[Marmosa (Marmosa)] beatrix Cabrera 1919:36. Name combination.
Marmosa agilis buenavistae Tate 1931:10. Type locality "Buenavista, Departamento Santa Cruz, Bolivia".
Marmosa agilis peruana Tate 1931:10. Type locality "Tingo Maria, Rio Huallaga" Huánaco, Peru.
Marmosa agilis peruania Tate 1933: pl.11. Incorrect spelling.
Marmosa blaseri A.Mirando.Ribeiro 1936:373. Type locality "S. Bento, Goias" Brazil.
Thylamys rondoni A.Mirando.Ribeiro 1936:387. Type locality "Salto do Sepotuba e São João da Serra do Norte", Matto Grosso, Brazil.
Marmosa [(Thylamys)] agilis Cabrera 1958:27. Name combination.
Marmosa [(Thylamys)] beatrix Cabrera 1958:27. Name combination.
Marmosa [(Thylamys)] buenavistae Cabrera 1958:27. Name combination.
Marmosa [(Thylamys)] peruana Cabrera 1958:28. Name combination.
[Thylamys] agilis Reig, Kirsch & Marshall 1987:7. Name combination.
Gracilinanus agilis Gardner & Creighton 1989:5. First use of current name.
ENG: Agile Mouse Opossum, Agile Opossum (Gardner 2007), Agile Gracile Mouse Opossum (Esquivel 2001, Cannevaro & Vaccaro 2007).
ESP: Marmosa ágil (Chebez 1996), Marmosa grácil ágil (Emmons 1999), Comadrejita rojiza (Massoia et al 2000), Comadrejita enana (Massoia et al 2000), Comadrejita ágil (Massoia et al 2000).
GUA: Anguyá-guaikí (Massoia et al 2000).
DES: A small, slender mouse opossum with short, smooth pelage and very short, inconspicuous guard hairs. Dorsally the pelage is brownish to greyish-brown, lacking patternation and often with a slightly grizzled effect. Ventral pelage buffy-white, with an indistinct greyish base to the hairs usually present and typically a line of self-coloured hairs along the midline of the body, stretching anteriorly to the throat and chin and posteriorly to the genital area. Head scarcely paler than dorsum with narrow black periocular patches, only slightly more conspicous than those of Cryptonanus. Males possess a gular gland. Ears moderately large and rounded, light grey-brown in colour. Vibrissae short. Feet pale pinkish. Calws on the manus do not extend beyond the digital pads. Palmar surfaces lack the a granular appearance, spare granules are present on the palmar surface. Tail brownish, lightly bicoloured (darker above and paler below) and 1.2-1.4x head and body length. It is prehensile, lacking hair on the ventral surface at the tip. Caudal scales are arranged in annular series and bearing sparse, almost invisible hairs. Females lack a pouch. Mammae are hidden when the female is not lactating. Abdominal-inguinal mammae 6-1-6 = 13, the most anterior reaching the thoracic region. (Tate 1933, Hershkovitz 1992). CR - Skull short and broad with pointed muzzle. Nasals moderately expanded basally. Palate long and strongly fenestrated. Zygomata expanded. Bullae large and rounded with distinct processes. Temporal ridges well-spaced. Supra-orbital ridges sharp-edged and with incipient processes. Maxillary palatal vacuities, rostral process of the premaxillae and a secondary foramen ovale all present, representing the primary distinguishing features from Cryptonanus. (Tate 1933, Voss, Lunde & Jansa 2005). DF: I5/4 C1/1 P 3/3 M 4/4 = 50. Incisors increase slightly in size from I2 to I5. P2 and P3 of approximately equal height, though be aware of the affects of teeth wear in older specimens. Canines short and close together. C1 accessory cusps are absent. Tooth rows convergent. M3 anterior cingulum complete. (Tate 1933, Gardner & Creighton 1989, Voss, Lunde & Jansa 2005).
TRA: No information.
MMT: A small Mouse Opossum. The species shows a clinal decrease in body size from north to south, with the smallest individuals occurring in Paraguay and northern Argentina. Bonvicino et al (2005) provided the following range measurements for 19 unsexed individuals from the cerrado of PN Chapada dos Veadeiros, Goias, Brazil: HB: 9.2-12.9cm; TA: 12.6-16.4cm; FT: 1.2-2.2cm; EA: 2.2-2.4cm; WT: 20-45g.
SSP: When using only external characters this species should be separated from Cryptonanus chacoensis with utmost care when using external characters only. Measurements and examination of skull characteristics may be necessary in some cases. Typically the tail of Cryptonanus is shorter when compared to head and body length (usually <1.2x) than that of Gracilinanus (1.2-1.5x) though there may be some overlap at the extremes and this character should not be used alone for specific designation. Tail length is typically in the range 95-117mm for adult Crytponanus and 110-165mm for Gracilinanus. More reliable is the ratio of premolar heights, with P2<P3 in Cryptonanus and the two of approximately equal height in Gracilinanus - though be aware of the affects of teeth wear in older specimens. On the canine C1 accessory cusps are present basally in Cryptonanus that are absent in Gracilinanus. Ventral pelage is usually somewhat greyish basally in Gracilinanus and buffy basally in this Cryptonanus. Upon direct comparison Gracilinanus has larger ears, longer vibrissae and broader ocular rings than Cryptonanus, but these characters are difficult to measure when presented with a single specimen. Cranially maxillary palatal vacuities, rostral process of the premaxillae and a secondary foramen ovale are all present in Graciliananus but absent in Cryptonanus. The species can be easily separated from the two species of Paraguayan Thylamys by the fact that members of that genus have distinctly tricoloured pelage, whereas Gracilinanus is uniformly-coloured dorsally. Thylamys also habitually exhibit some degree of incrassination (fat deposits) in the tail and have highly granular surfaces to the feet, neither character being exhibited by this species. Furthermore the species occurring in eastern Paraguay, Thylamys macrurus, is considerably larger than Gracilinanus. Micoureus paraguayanus is much larger with thick woolly pelage and broadly pale-tipped, bicoloured tail. (Voss, Lunde & Jansa 2005). A second species of Gracilinanus, G.microtarsus, is of hypothetical presence in the Atlantic Forest of Paraguay. It can be distinguished by its uniform reddish-brown to chestnut-brown pelage as compared to grizzled greyish-brown in G.agilis. Crucially the ventral pelage of microtarsus is almost entirely grey-based except for chin, that of agilis is not grey-based on the chin, throat, upper breast and scrotal area. G.microtarsus has a notably blacker and more extensive ocular patch which extends to nose and ears and the face is contrastingly paler than dorsum. Morphometrically the tail is typically >140mm and ears usually <21mm, those of G.agilis usually <140mm and >21mm respectively (Costa et al 2003).
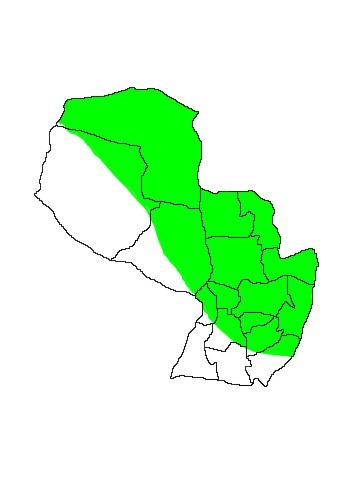
DIS: Widely distributed from northern and eastern Peru, through northern and eastern Bolivia and Paraguay to Brazil. In Brazil the distribution carves a wide arc south of the Amazon Basin and it has been recorded in the states of Maranhão, Ceará, Tocantins, Distrito Federal, Minas Gerais, Goias, Matto Grosso and Matto Grosso do Sul (Gardner 2007, Cáceres et al 2008). The species is apparently replaced by Gracilinanus microtarsus in the coastal Atlantic Forest from Minas Gerais (where they overlap slightly) to Rio Grande do Sul (Brown 2004). The text description of the range provided by Gardner (2007) does not correspond to the range map provided, mentioning Uruguay and adjacent Argentina but not mapping any points south of Asunción, Paraguay. Massoia et al (2000) map the species south to Provincia Buenos Aires, but their Gracilinanus agilis includes the recently recognised Cryptonanus chacoensis which apparently occurs much further south. Flores (2006) considers all records of Gracilinanus agilis in Argentina to refer to Cryptonanus chacoensis, but Chebez (2009) called for a review of all known specimens believing that the species may be present in Provincia Misiones. In Paraguay the species is apparently widely-distributed in forested areas, though its precise distribution is confused by its wide sympatry with the cryptic Cryptonanus chacoensis. Five specimens collected at Sapucaí, Departamento Paraguari are in the British Museum (BMNH 3.2.3.39, 3.4.7.22, 3.4.7.23, 4.1.5.46 and 4.1.5.47).
HAB: Occurs in forested areas, typically dry. In northern Paraguay it is found in cerrado and occurs in chaco woodland in the Chaco. Of 19 individuals in the cerrado of PN Chapada dos Veadeiros, Goias, Brazil, 11 were collected in cerrado sensu strictu and 7 in cerradón, but none were trapped in gallery forest (Bonvicino et al 2005).
ALI: Insectivorous, frugivorous and carnivorous. They relish sweet, juicy foods and the weak dentition means they prefer soft, pulpy items. Whilst they are incapable of breaking bark to feed on exudate, they will take advantage of the work of other animals and feed on leaking sap etc. Individuals have been caught in traps baited with banana pulp and peanut butter. (Hershkovitz 1992). Astúa de Morães et al. 2003 experimentally tested the proportions of protein, lipid, carbohydrate and fibre in the diet of adults (n=5) and juveniles (n=2) of this species under laboratory condtions. Mean proportions per 100g dry weight of food were: protein ad. 1.34g (+/-0.97), juv. 1.00g (+/-1.30); lipid ad. 0.32g (+/-0.32), juv. 0.87g (+/-1.21); carbohydrate ad. 3.41g (+/-0.90), juv. 3.09g (+/-2.28); fibre juv. 3.18% (+/-0.72). Santori et al (2004) described and illustrated the gut morphology of this species and associated it with dietary habits.
REP: Breeding seasonal according to the availability of resources, with one or two breeding seasons per year. Females were not carrying pouched young during August in PN Chapada dos Veadeiros, Goias, Brazil (Bonvicino et al 2005) and juveniles have been found in Bolivia during April and December (Tate 1933). Litters consist of up to 12 young (Eisenberg & Redford 1999).
BEH: Activity Levels Nocturnal, solitary and arboreal being most frequently trapped in the understorey amongst viney tangles and thin branches at a height of 1.5-2m. Locomotion The species moves along thin branches and vines with short, rapid steps interchanged with overhand climbing when moving up and down. The hands and prehensile tail are used for grappling. The claws of the forefeet are weak, but those of the hind feet are stronger and are used to sustain the animal when climbing downwards head first. Individuals have been seen to use the tail to swing slightly when climbing down from precarious vines. (Hershkovitz 1992). The species occasionally descends to the floor where it is slow-moving and easily captured by hand (P.Smith pers.obs.). Roosts Nests are built from grasses and vegetable fibres in low bushes or tree holes at a height of about 1.5m. (Canevari & Vaccaro 2007). Defensive Behaviour Hershkovitz (1992) described two individuals of this species which reacted to capture by raising up on their hind legs with forearms outstretched and palms facing forwards, mouth open wide exposing the dentition and making hissing sounds. When prodded with a stick the animals grasped it with the forefeet and bit it weakly. Enemies Hershkovitz (1992) lists snakes, owls and lizards, as well as "any large predator large enough to gulp down a mouse-size morsel". A low rate of trap mortality has been observed due to the ability of this species to enter into a torpid state when cold. (Hershkovitz 1992). Parasites Limardi (2006) listed the following ectoparasites from Brazilian specimens: Acari: Metastigmata Amblyomma cajennense and Ixodes sp. (Ixodidae). Acari: Mesostigmata Bdellonyssus sp. (Macronyssidae). Acari: Astigmata Didelphoecius palmeirensis (Atopomelidae).
VOC: Animals in defensive posture make hissing sounds (Hershkovitz 1992).
HUM: None.
CON: Globally considered to be of Low Risk Least Concern by the IUCN, click here to see their latest assessment of the species on account of its wide distribution, large population and occurrence in protected areas. Though rarely observed, trapping studies show that this species is fairly common in forested areas. The main threat to the species is likely to be through conversion of forest habitat to agriculture and ranchland. However Henriques et al (2006) studied the small mammal populations in areas of cerrado in various stages of regrowth after fire and found the species to be much more numerous in areas that had not been burned for more than 12 years, suggesting that regular burning may negatively affect populations.
Citable Reference: Smith P (2009) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 35 Gracilinanus agilis.
Last Updated: 30 June 2009.
References:
Anderson A 1997 - Mammals of Bolivia: Taxonomy and Distribution - Bulletin AMNH 231.
Astúa de Morães D, Santori RT, Finotti R, Cerquiera R 2003 - Nutritional and Fibre Contents of Laboratory-established Diets of Neotropical Opossums (Didelphidae) p225-233 in Jones M, Dickman C, Archer M Predators with Pouches: The Biology of Carnivorous Marsupials - CSIRO Publishing, Australia.
Bonvicino CR, Lemos B, Weksler M 2005 - Small Mammals of Chapada dos Veadeiros National Park (Cerrado of Central Brazil): Ecologic, Karyotypic and Taxonomic Considerations - Brazilian Journal of Biology 65: p395-406.
Brown BE 2004 - Atlas of New World Marsupials - Fieldiana Zoology 102.
Burmeister H 1854 Systematische Uebersicht der Thiere Brasiliens: Welche während einer Reise durch die Provinzen von Rio de Janeiro und Minas Geraës Gesammlt oder beobachtet wurden Vol 1 - G.Reimer, Berlin.
Cabrera A 1919 - Genera Mammalium. Monotremata, Marsupialia - Museo de Ciencias Naturales, Madrid.
Cabrera A 1958 - Catálogo de los Mamíferos de América del Sur - Revista Museo Aregntino de Ciencias Naturales Bernadino Rivadavia Zoology 4: p1-307.
Cáceres NC, Casella J, Vargas CF, Prates LZ, Tombini AAM, Goulart CS, Lopes WH 2008 - Distribucão Geográfica de Pequeños Mamíferos Não Voadores nas Bacias dos Rios Araguaia e Paraná, Região Centro-Sul do Brasil - Iheringia Serie Zool. 98: p173-180.
Cannevari M, Vaccaro O 2007 - Guía de Mamíferos del Sur de América del Sur - LOLA, Buenos Aires.
Chebez JC 1996 - Fauna Misionera - LOLA, Buenos Aires.
Chebez JC 2009 - Otros que Se Van - Editorial Albatros, Buenos Aires.
Costa LP, Leite YLR, Patton JL 2003 - Phylogeography and Systematic Notes on Two Species of Gracile Mouse Opossums, Genus Gracilinanus (Marsupialia: Didelphidae) from Brazil - Proceedings of the Biological Society of Washington 116: p275-292.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Esquivel E 2001 - Mamíferos de la Reserva Natural del Bosque Mbaracayú, Paraguay - Fundación Moises Bertoni, Asunción.
Flores DA 2006 - Orden Didelphimorphia in Bárquez R, Díaz, MM, Ojeda RA eds Mamíferos de Argentina, Sistemática y Distribución - SAREM, Buenos Aires.
Gardner AL 2007 - Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats - University of Chicago Press.
Gardner AL, Creighton GK 1989 - A New Generic Name for Tate´s microtarsus Group of South American Opossums (Marsupialia: Didelphidae) - Proceedings of Biological Society of Washington 102: p3-7.
Henriques RHB, Briani DC, Palama ART, Vieira EM 2006 - A Simple Geographical Model of Small Mammal Succession After Fire in the Brazilian Cerrado - Mammalia 70: p266-230.
Hershkovitz P 1992 - The South American Gracile Mouse Opossums Genus Gracilinanus Gardner & Creighton 1989 (Marmosidae: Marsupialia): A Taxonomic Review with Notes on General Morphology and Relationships - Fieldiana Zoology Series 1441.
Limardi PM 2006 - Os Ectoparasitos de Marsupiais Brasileiros p27-52 in Cáceres NC, Monteiro-Filho ELA Os Marsupiais do Brasil:Biologia, Ecologia e Evolução - Editora UFMS, Campo Grande.
Massoia E, Forasiepi A, Teta P 2000 - Los Marsupiales de la Argentina - LOLA, Buenos Aires.
Matschie P 1916 - Bemerkungen über die Gattung Didelphis L. - Sitzungsber. Gesells. Naturf. Freunde Berlin 1916: p259-272.
Mirando-Ribeiro A de 1936 - Didelphia ou Mammalia-Ovovivipara. Marsupiaes, Didelphos, Pedimanos ou Metatherios - Revista Museu Paulista 20: p245-427.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Reig OA, Kirsch JAW, Marshall LG 1985 - New Conclusions on the Relationships of the Opossum-like Marsupials with an Annotated Classification of the Didelphimorphia - Ameghiniana 21: p335-343.
Santori RT, Astúa de Moraes D, Cerqueira R 2004 - Comparative Gross Morphology of the Digestive Tract in Ten Didelphidae Marsupial Species - Mammalia 69: p27-36.
Tate GHH 1931 - Brief Diagnoses of Twenty-six Apparently New Forms of Marmosa from South America - AMNH Novitates 493.
Tate GHH 1933 - A Systematic Revision of the Marsupial Genus Marmosa - Bulletin AMNH 66.
Thomas O 1910 - On Mammals Collected in Ceará, NE Brazil by Fräulien Dr Snethlage - Annals and Magazine of Natural History Series 8 6: p500-503.
Voss RS, Lunde DP, Jansa SA 2005 - On the Contents of Gracilinanus Gardner & Creighton 1989 with the Description of a Previously Unrecognised Clade of Small Didelphid Marsupials - AMNH Novitates 3482.
ACKNOWLEDGEMENTS
Special thanks to Juan Carlos Chebez for providing important literature and Nilton Cáceres for very kindly reviewing texts and providing a copy of his book Os Marsupiais do Brasil.
MAP 35:
Gracilinanus agilis


